Editor’s note: This article has been updated August 2021 to reflect the current state of the COVID-19 vaccines in the FDA approval process.
Vaccination is the process of protecting a person from disease by making him or her immune. Researchers often do this by delivering a small piece of the organism that mimics the disease—called an antigen—to the person. That person’s immune system then makes antibodies to the organism, just like it would against any foreign substance. Although simple in concept, vaccine development takes time and money.
“Because vaccines are given to prevent disease and not treat it, they are usually administered to healthy people, so the standards for safety are higher than other drug classes,” said Dr. Thomas Boyce, pediatric infectious disease doctor at Marshfield Children’s and clinician researcher at Marshfield Clinic Research Institute. “In addition, there is often a fine balance in creating a vaccine that is strong enough to induce an immune response but not so strong as to cause side effects. Thus, vaccines are some of the most highly regulated pharmaceuticals.”
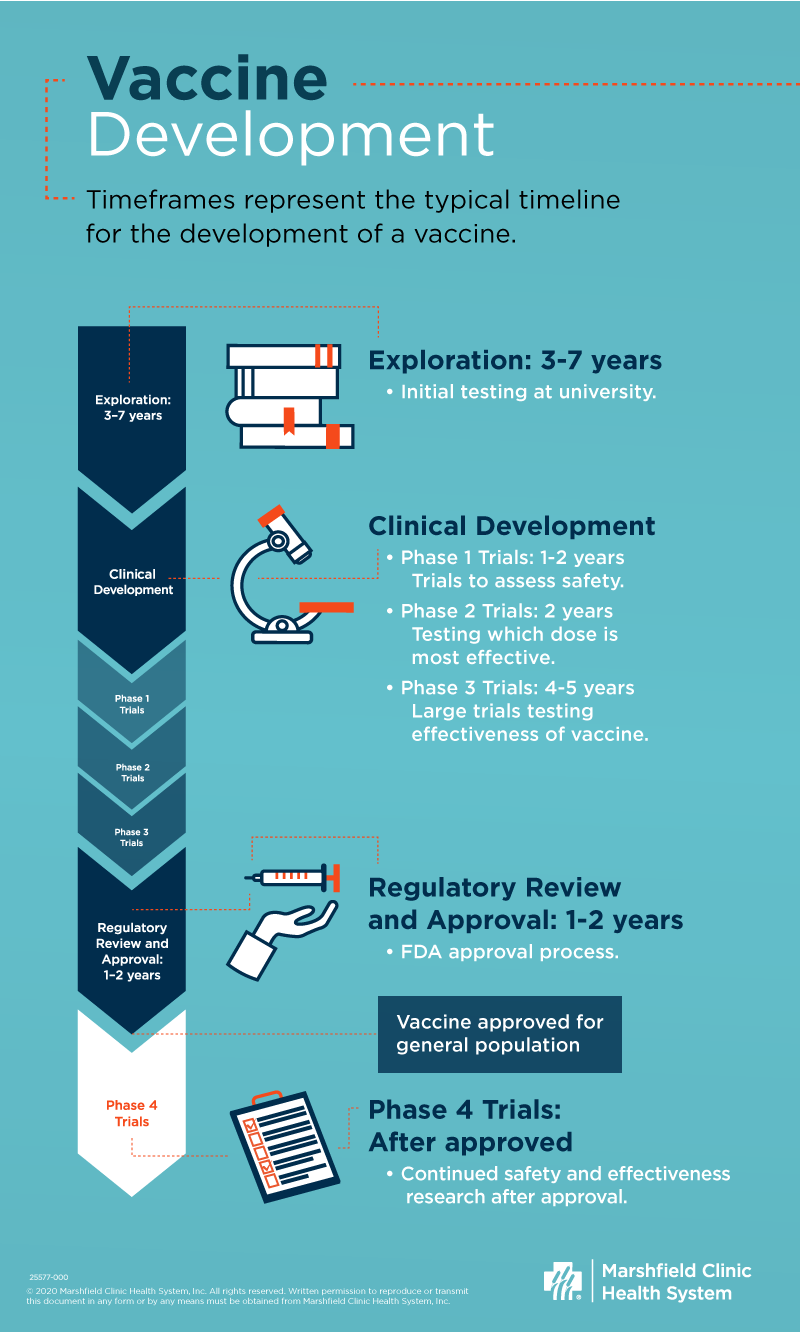
The development of a vaccine proceeds through several stages: exploration, clinical development, and regulatory review and approval. At each stage, there are continuous assessments of manufacturing and quality control.
Exploration: 3-7 years
The initial steps of vaccine development usually take place in a university research laboratory with funding by the federal government. This may include testing the investigational vaccine in small animals, such as mice. If the preliminary data appear promising, collaboration with a vaccine manufacturer may occur. This stage may take a few to several years, depending on the vaccine.
Clinical development: 7-9 years
Clinical development includes four stages of clinical trials:
- Phase I trials test the vaccine in a small number of healthy adults. These trials generally include less than 100 people, mainly assess safety and typically take one to two years.
- Phase II trials involve a larger number of people, usually 100-200 people. Researchers continue to assess safety, but they also find which dose works best. Phase II trials generally take at least two years.
- Phase III trials are large, placebo-controlled trials, often including tens of thousands of people. While researchers continue to look at safety, the primary goal is to determine how well the vaccine works, or efficacy. Phase III trials can take several years, often four or five.
- Phase IV trials are post-marketing trials. Researchers conduct these large studies after the vaccine approval to look for rare side effects and effectiveness in specific populations. Marshfield Clinic Research Institute performs Phase IV safety monitoring for many vaccines, including COVID-19 vaccines.
Regulatory review and approval: 1-2 years
The vaccine manufacturer must apply to the Federal Drug Administration (FDA) for a biologics license application (BLA). The FDA’s Center for Biologics Evaluation and Research (CBER) is responsible for licensing of the vaccine. The FDA requires laboratory testing for vaccine purity and consistency both before and after licensure. It inspects the manufacturing facility where the vaccine manufacturer produces the vaccine. The FDA also reviews all data from all clinical trials of the vaccine. This process typically takes 1-2 years.
“Vaccine development typically takes about 10-15 years, if all goes well. It costs hundreds of millions of dollars to develop a vaccine, and only about one in 15 vaccines that are entering Phase II trials ever becomes licensed,” Dr. Boyce said.
Vaccine development is a collaboration between academia, the government and the pharmaceutical industry. However, it is primarily pharmaceutical companies that incur the financial risk. We appreciate the ingenuity and perseverance of people who develop vaccines as vaccines are one of the best tools we have to prevent illness.
Process for COVID-19 vaccine
Although COVID-19 vaccines became available much faster than the typical timeline listed above, it is important to realize that they were studied just as thoroughly as any other vaccine. Dr. Boyce explains the reason COVID-19 vaccines were available so quickly is because the vaccine companies did not wait to manufacture large quantities of vaccines until after they were approved.
“In some cases with government funding, they manufacture the vaccines while they are under review so they would be available much sooner,” he said.
Three COVID-19 vaccines are currently available under an emergency use authorization from the FDA.
“The timeline from discovery of the virus to development of available vaccines in about a year is remarkable. This was the result of unprecedented effort of scientists and investment by the government in funding the manufacturing of the vaccines in anticipation of their approval,” Dr. Boyce said.
A fully FDA approved product can be sold directly from a manufacturer to health care entities. All three COVID-19 vaccines Janssen (Johnson & Johnson), Pfizer and Moderna have met FDA approval standards for safety, effectiveness and manufacturing quality. Therefore, each submitted for full approval and are under priority review. FDA approval will allow direct sales and safety and effectiveness testing will continue after approval.
For more information about vaccines, talk to your health care provider.
Vaccine development typically takes about 10-15 years. It costs hundreds of millions of dollars to develop a vaccine, and only about one in 15 vaccines that are entering Phase II trials ever becomes licensed.


Karen G. My daughter has a rare disease, Rhett syndrome. We have a scheduled appointment to get the vaccine for Covid-19 in February. Is there any information known about people with Rhett syndrome and any side effects from the vaccine that I should be concerned?
Hello Karen,
Thank you for reaching out.
The vaccine clinical trials did not assess vaccine safety or efficacy among people with Rhett syndrome. However, people with certain types of inherited or neurologic disease have increased risk for severe Covid-19. Clinical trials have shown that vaccination is highly protective among all adult age groups, and in people with and without common chronic diseases. The CDC has made an interim recommendation for use of Covid-19 vaccines in all adults age 18+ years (Moderna) or 16+ years (Pfizer/BioNTech). We recommend talking to your doctor about any special considerations for people with Rhett syndrome.
Hope that helps,
Jake
I was told by a Marshfield employee that you need to fill out a survey to get your name on a list to be called for the vaccination. What is the link to fill out that survey? I am asking for my dad who is 86 years old.
Hi Jody,
The COVID-19 vaccine is not available at this time for most patients. The Wisconsin Department of Health Services and CDC have prioritized front line health care workers, first responders and residents of long-term care facilities as the first to receive the vaccine. We are expecting further guidance from the state about the rollout of the vaccine beyond these groups in the coming weeks. We will communicate out to the community once the vaccine is available. You can also watch our COVID-19 hub at marshfieldclinic.org/coronavirus for the latest updates. We appreciate your patience as we continue to plan for mass vaccination in the coming months.
Thank you,
Jake
I am 63 years old, have asthma and diabetes. How will I know when it is “my turn” to get the Covid vaccine? Will it be administered at Marshfield Clinic?
Hi Jean,
Thank you for reaching out. The COVID-19 vaccine is only available to frontline health care workers at this time. We will communicate to the public once it is available to additional groups. If you would like to stay up to date and read more, please feel free to check out our online COVID hub: https://www.marshfieldclinic.org/specialties/infectious-diseases/coronavirus-update
We have a variety of resources here that may be helpful.
-Jake
Hello, my husband (77 y.o.) and myself (71 y.o.) have not heard of any timeline as to when we may able to receive the Vaccine Shot. We live in the Minocqua area and just haven’t heard any news concerning the Vaccine. Do you have any more information.?
Hi Kathy,
Thank you for reaching out. The COVID-19 vaccine is only available to frontline health care workers at this time. We will communicate to the public once it is available to additional groups. If you would like to stay up to date and read more, please feel free to check out our online COVID hub: https://www.marshfieldclinic.org/specialties/infectious-diseases/coronavirus-update
We have a variety of resources here that may be helpful.
-Jake
I am allergic to Methotrexate and Flagile. I am not supposed to have vaccines that use live viruses. Will I be able to get the Covid vaccine?
Hi Margarette,
The CDC’s Advisory Committee on Immunization Practices has not made any recommendations yet on who should or should not receive the COVID-19 vaccine. So we cannot recommend whether you should take the vaccine at this time. Make sure to talk to your doctor before you receive the vaccine. However, we can tell you that none of the vaccines in clinical trials include live virus.
Thanks
Richard
I am 73 and my wife is 70.
We plan to spend the winter in Florida.
If a COVID-19 vaccine becomes available will we be able to get that vaccine in Florida being our home is in Wisconsin?
Hello Richard – thank you for reaching out,
Our best recommendation would be to reach out to your local public health officers in either Florida or Wisconsin to inquire about timelines and your specific situation as distribution of vaccines may differ upon states or even counties at least initially.
Thanks,
Jake